Gastroenterology
The US FDA has issued an early alert concerning some Axios stent and delivery systems used in endoscopic drainage procedures after receiving reports of serious injuries and deaths linked to the device.
Last year was a busy one for the medtech industry, with major advances in technology as well as big changes at regulatory agencies. Get a peek at the stories our readers couldn't miss.
Shantanu Gaur, CEO of Allurion Technologies, a company that has developed a swallowable gastric balloon, believes that GLP-1 sales could indirectly lead to increased demand for medical device-based bariatric procedures, especially as patients seek alternatives to drug side effects and weight regain.
The US FDA says Olympus has updated its instructions for a device used in many endoscopic procedures after reports of serious injuries. The class I recall follows the FDA blocking imports of other scoping devices from the Japanese firm earlier this year.
Inspired by his son’s battle with cancer, Neal Piper founded Luminoah to modernize enteral feeding. Its smart, pocket-sized pump tracks nutrition in real time to prevent malnutrition and improve quality of life for patients and caregivers.
At the MedTech Conference, the CEOs of Stryker, Hologic and Insulet said during a panel AI is now central to their business strategies, from creating new medical devices to streamlining workflows and administrative tasks. Stryker’s CEO Kevin Lobo plans a business unit focused on AI-enabled tech.
Mendaera’s Handheld Robotic System Poised To ‘Transform Needle-Based Procedures’ Starting In Urology
After receiving US FDA clearance and reporting first successful use at Mayo Clinic, the Silicon Valley start-up plans to partner with leading centers to transform needle-based procedures with its robotic system starting with urology, then expand into other specialties, the CEO said.
SafeHeal has secured an additional €10m for its Colovac device, bringing its total funding to €97.5m. Led by Asabys Partners, this funding offers both economic and clinical benefits, potentially reducing costs and complications associated with temporary stomas.
Microbot wins FDA nod for Liberty, Medtronic’s Hugo showed positive results in hernia repair in clinical studies, Obvius Robotics reports first human procedure with CERTA, Ronovo closes a $67m financing round with JJDC, and Quantum expands Epione CE marking to bone tumors.
Salient's inflammatory bowel disease test, built on the start-up’s Signal platform, is planned to launch in March 2026. The company leverages rich data from existing wellness tests to develop signatures, focusing on conditions that are often misdiagnosed and disproportionately affect women.
Roche Diagnostics, Intuitive Surgical, Abbott and Edwards Lifesciences all acknowledge tariff headwinds, but stress preparedness, resilience and mitigation. Below is look at how management framed the impact in Q1 vs Q2 earnings calls.
The US FDA has provided an update on its breakthrough devices program, now 10 years running. Cardiac, orthopedic, and neurological devices have received the most designations, while only one has been awarded to an obstetrics/gynecology product.
Olympus and Revival Healthcare Capital have launched Swan Endosurgical in a $458m milestone-based joint venture to develop a robotic platform for endoluminal surgery. The deal combines Olympus’ GI expertise with Revival’s start-up model, but will face technical and regulatory hurdles.
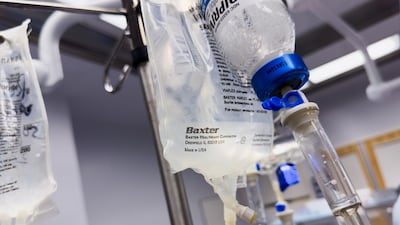
The US FDA says Baxter has notified customers about an issue with its Novum IQ Syringe Pump that is linked to two deaths and multiple injuries.
CEO Alexandre Chau told Medtech Insight that the Middle East and Africa presented the ideal mix of market potential, physician training opportunities, and regulatory readiness when the company was considering expansion markets beyond Europe and the US.
Researchers at Johns Hopkins have used a robotic system to autonomously perform a key part of gallbladder surgery without a surgeon's hand. Lead author Axel Krieger says it could take five to 10 years before an autonomous robotic system will reach human trials and expects regulatory hurdles.
The US FDA has issued an early alert to notify consumers about serious risks associated with a recall from Medtronic and its subsidiary Given Imaging. The alert concerns delivery capsules for diagnosing GERD.
SS Innovations reported its first SSi Mantra 3 robotic cardiac surgery in South America as it prepares to file for US FDA de novo clearance and a CE mark. The firm aims to challenge Intuitive Surgical with a lower-cost, flexible system targeting US and European markets.
SS Innovations announced plans to file a de novo application with the US FDA for its SSi Mantra 3 surgical robot, already approved for marketing in six countries. The Florida-based firm also plans to pursue the CE mark for European commercialization.
Global investment in consumer healthtech increased by 9% year-over-year in 2024, totaling $4.5bn, with significant interest in mental health solutions, according to Galen Growth. While the first quarter of 2025 saw raised confidence and investments, the Trump administration’s new tariffs and sweeping changes to healthcare have introduced new uncertainties.