United Kingdom
With EU MDR/IVDR review proposals out, UK MHRA progress on a premarket regulation and, up ahead, a prospective consultation on accepting the CE marking indefinitely, now is a good time to assess EU-UK regulatory positions for the benefit of UK industry, says ABHI director Steve Lee.
The UK MHRA will soon consult on allowing CE-marked devices indefinite access to the Great Britain market. Meanwhile, the EU has at last moved to make the MDR/IVDR more user friendly. Taylor Wessing partner Alison Dennis reflects on how these events could influence evolving UK medtech legislation.
The UK health care products regulatory agency is inviting industry to share its views on how artificial intelligence in health care should be regulated, with input set to shape future rules and guidance.
A forward-looking view of prospects for regulatory and market access improvement from the UK’s biggest medtech industry lobby group, the Association of British HealthTech Industries (ABHI).
Joint Singapore-UK plan underlines direction of travel for MHRA after its embracing of international reliance (IR). Australia’s TGA has also broken new ground by recognizing the UKCA mark.
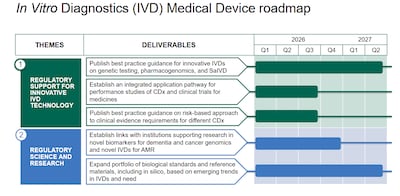
Roadmap version 1.0 is a living document, not a legislative instrument, that will be updated and tweaked for timings as necessary.
Digital technology and AI have pushed healthcare delivery to a transformational turning point, but market access, budget constraints and scaling adoption of innovation remain the eternal challenges for healthtech manufacturers.
The MHRA’s premarket statutory instrument for Great Britain should to be laid before UK parliament in Q2 and be in force by year end, in an ideal world. There are concerns that the new statutory instrument must align with imminent EU MDR changes.
The UK Medicines and Healthcare products Regulatory Agency said its RegulatoryConnect program “no longer offers value for money for UK taxpayers” and will be closed just 19 months after its April 2024 launch.
The prosthetics market is evolving due to an increase in amputees from war and disease. Unhindr has developed a novel prostetic liner that can reduce the risk of further injury, while Esper is crafting advanced hand implants for Ukraine's front-line soldiers.
The first-of-its-kind sandbox for insights on how to regulate artificial intelligence in healthcare has accepted seven more AI innovators onto its learning program.
Market and regulatory support for 117 new and 91 existing devices under 2024-25 technology funding program run by UK innovation agency CPI benefits 141 SMEs – but demand for regulatory support outstrips supply.
June Raine was a “hard act to follow” at MHRA, but new chief executive Lawrence Tallon is looking to the future in setting out a vision for the UK regulator.
Evidence-based testing in real clinical settings and diagnostics tailored to community needs are crucial adoption success factors for new IVDs, Michael Wright, joint medical director at Newcastle Hospitals NHS Foundation Trust, told Medtech Insight at a regional UK meeting.
At LSX World Congress USA, medtech executives shared lessons on global expansion, from regulatory pitfalls to cultural nuances and funding gaps. Their message: Prepare early, secure capital, and choose partners wisely.
Sava Technologies has developed a CGM that uses microneedles to accurately measure glucose levels for up to 10 days without finger-prick calibration. The company raised £14.2m in Series A funding to establish in-house manufacturing for faster production.
MHRA changes how it will charge device companies for post-market surveillance work based on GMDN codes registered to the relief of SMEs.
Salient's inflammatory bowel disease test, built on the start-up’s Signal platform, is planned to launch in March 2026. The company leverages rich data from existing wellness tests to develop signatures, focusing on conditions that are often misdiagnosed and disproportionately affect women.
The UK government expects that its new nation-wide network will enable companies to set up clinical trials more quickly and expand access to diverse patient populations across the National Health Service.
The ceramic device, designed to reduce complications, will be exclusively distributed by Zimmer Biomet and initially available in 30 hospitals, with broader European access expected by 2026.