Drug Pricing
The US-India dance around pharma goes on as they announce a trade deal framework that continues to exempt generics from the reduced 18% tariffs till a “negotiated outcome” is reached post a Sec. 232 investigation. However, oil imports from US could increase costs for Indian pharma firms
The Trump administration has launched TrumpRx, a website the White House says could help save Americans billions in pharmaceutical spending, although it does not itself sell or dispense drugs.
Novo Nordisk said it will take legal action against mass compounding of the new oral semaglutide formulation by Hims & Hers.
New CEO Mike Doustdar believes Novo Nordisk can win in the lower-price, higher-volume consumer market for obesity drugs – but is nonetheless preparing investors for a hit to sales and profits in 2026.
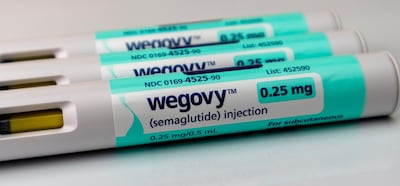
The Danish firm’s share price plummeted as it revealed its most favored nation pricing deal would cut deep into sales and profits in 2026, although offset somewhat by strong launch of the Wegovy pill.
The process will pit breast cancer treatment competitors Kisqali and Verzenio against each other as CMS develops pricing offers.
Conclusion of India-EU talks sets ground for a free trade agreement to cut pharma tariffs, in turn lowering the cost for novel drugs like Novo’s obesity treatment Wegovy, though an investment protection agreement – likely influencing data exclusivity – will be concluded later.
Despite group sales growth and its pharmaceutical division revenue weathering a big loss of exclusivity, J&J experienced stock price weakness after its fourth-quarter results announcement as geopolitical tensions depressed stock markets again.
Industry leaders predict pricing uncertainty will force companies to abandon blanket global launches. However, AI platforms will reshape commercial execution and new access models will break down barriers limiting patient reach.
BMS tells Scrip it will continue to seek legal recourse even as Zydus Lifesciences launches the world’s first cut-price nivolumab biosimilar in India following a Delhi High Court division bench ruling in favor of the Indian company
The Swiss pharma co-authored a “call for bold life sciences investment” with the Eurasia Group, critiquing global trade policy and advocating “innovation-friendly” reforms, including moving away from reference pricing.
The plan does not include many details but is intended to help Congress draft legislation ensuring Most Favored Nation drug pricing agreements can be made, as well as implement measures intended to lower insurance premiums.
The announced deal means that of 17 companies the White House notified in July, only AbbVie and Regeneron have not yet made a deal.
With a starter dose price of $149 a month for self-payers, oral Wegovy will be available directly from Novo Nordisk and through telehealth providers and large pharmacy chains.
There has not been a dull moment in the biopharma sector this year. With just a day left, and in no particular order, Scrip takes a look at five of the biggest stories of 2025.
Industry veterans put themselves in Novo’s shoes, suggesting pricing manoeuvres and tactical measures to blunt competition in India post semaglutide’s LOE in 2026. There’s also court action to watch.
Amgen, BMS, Boehringer Ingelheim, Genentech, Gilead Sciences, GSK, Merck & Co., Novartis and Sanofi will reduce prices of some drugs for Medicaid and offer reduced pricing through direct-to-patient platforms, among other concessions. In exchange, they will be exempted from tariffs.
Ozempic debuts in India, priced in the ‘affordable zone’ for the country's population, with Novo Nordisk hoping the blockbuster drug can hold its own post impending loss of exclusivity in the country next year.
Sun launches tildrakizumab, its star psoriasis therapy in India seven years after US FDA approval at what’s seen as a carefully calibrated price point. Can it ruffle entrenched products like secukinumab?
The Trump administration leveraged tariffs to convince the UK National Health Service to increase prices for new medicines by 25%.