Switzerland
As 2025 turns to 2026, Swiss medtechs are anxiously looking to the future on three major fronts, according to Daniel Delfosse, head of regulation and innovation at the national industry association, Swiss Medtech.
Swiss medtechs have given overwhelming support for an ongoing bilateral approach between Switzerland and the EU, as proposed in the Swiss-EU Package in June. A medtech MRA is the ultimate prize, but that remains some way off.
The UDI devices module in swissdamed is now live and can be used on a voluntary basis until it becomes mandatory in mid-2026.
In return for building a successful US export business, Switzerland has been handed a 39% tariff effective immediately by the Trump administration. Diplomatic channels have been reopened to undo the tariff. Meanwhile, the medtech industry is looking to develop OUS markets for Swiss exporters.
The US’ 90-day suspension of higher “reciprocal tariffs” will expire on 9 July, raising fears that the 31% tariff rate on Swiss medtech and other goods exported to the US might make a comeback. While the tariff situation changes on an almost daily basis, medtechs should forearm for any eventually, say local business organizations.
Its move comes as the Swiss recognize the need to ensure adequate supplies on medical devices in its country.
The medtech sector in the UK, Switzerland, and Germany face a challenging 2025, heads of national trade groups say. Competition and access to innovation remain key concerns across the board, with collaboration with the EU and US planned in Switzerland while Germany focuses on approaching elections.
Medtechs will be indirectly affected by a new law in Switzerland that unifies the amount of funding allocated to inpatient and ambulatory care.
Switzerland’s devices MRA with the EU is fully back on the agenda following a broader, historic EU-Swiss future trade deal agreed on 20 December. Additionally, Swiss Medtech is pursuing MDR improvements with EU counterparts, and the Swiss authorities will lay the ground in 2025 to permit US FDA-approved in Switzerland.
An agreement between the EU and Switzerland potentially opening the way for renewal of the MRA – enabling barrier-free cross-border medtech trade – could be concluded as early as December. More good news for Switzerland, relating to the acceptance of FDA-approved products into the country, could follow in Q1 2025.
Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Over 40 documents have been posted on the tracker since its last update.
Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Nearly 80 documents have been posted on the tracker since its last update.
Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Nearly 30 documents have been posted on the tracker since its last update.
Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Over 50 documents have been posted on the tracker since its last update.
Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Sixty-five documents have been posted on the tracker since its last update.
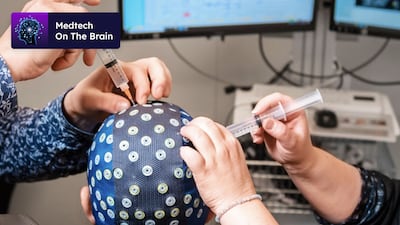
Swiss-based start-up DeepPsy aims to streamline mental health care using EEG and ECG biomarkers to better match depression patients with treatments. Co-founder Mateo de Bardeci discusses the company’s vision as it launches its in-house medical device as a service in Switzerland.
Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Thirty-five documents have been posted on the tracker since its last update.
Switzerland has been developing a national medical devices database to mirror Eudamed, the EU version that has excluded participation by Swiss companies since the failure of the federal council and European Commission to update the MRA for devices.
Negotiations aimed at renewing the close trading relationship and barrier-free mutual market access between Switzerland and the EU got underway on 19 March. Swiss medtech companies welcomed the move, stressing the need for the defunct mutual recognition agreement to be updated.
Stay current on regulatory guidelines from around the world with Medtech Insight's Guidance Tracker. Thirty-six documents have been posted on the tracker since its last update.