Neurology
Cerenovus is recalling multiple products from its Cerepak Uniform, Cerepak Heliform and Cerepak Freeform product lines due to a higher-than-expected failure to detach rate. While customers were sent a safety alert in October, the US FDA announced the recall on Feb. 5.
INBRAIN unveiled a bidirectional "rice-sized" BCI chip partnership, Merck commercialization progress and new speech-decoding trial in France as it advances its graphene-based cortical interface toward commercialization, pending regulatory clearance.
Medtech Insight was invited to moderate a panel discussion with leading experts in neuroscience and AI during INBRAIN’s five-year anniversary in Barcelona, Spain. Panelists discussed the promises, perils in BCI development, neuroethics and outlook.
In this final part of a three-part series, Medtech Insight spoke with a neuroethicist and the first person in a trial using a BCI implant for stimulating hand movement. This story explores ethical considerations that arise when projects can no longer support patients with implanted devices.
The electrostimulation pain treatment firm reportedly shipped excessive supplies to patients for eight years, driving up revenues as well as stock prices.
After receiving the FDA nod for the first in-ear EEG device for brain monitoring, Noax plans four clinical trials in the US focused on epilepsy and is looking for audio company partners to integrate the same tech for use by consumers to monitor their brain activity, the company told Medtech Insight.
MIT-scientist turned entrepreneur Christin Glorioso founded start-up NeuroAge Therapeutics to reverse brain aging and fight dementia.
In this first roundup from CES, Medtech Insight shines a light on AI-powered technologies that help the aging population live healthier and stay independent longer.
In this second of a three-part series, Medtech Insight spoke with three neuroethicists who raised concerns about privacy, patient safeguards and the need for comprehensive guidelines. These issues are becoming more pressing as BCI companies get ready to commercialize their products.
Rapid advancements in neurotechnology intensify the need for clear regulations concerning neural data privacy. As these technologies evolve at unprecedented speeds, lawmakers, legal experts, and neuroethicists are increasingly focused on their societal impact.
Neurotech start-up Subsense raised another $10m, bringing its total seed funding to $27m. The proceeds will be used primarily to accelerate and enhance the company’s pre-clinical research program.
The US FDA has approved Flow Neuroscience’s at-home brain stimulation device for the treatment of depression, ending a frustrating wait for the Swedish firm.
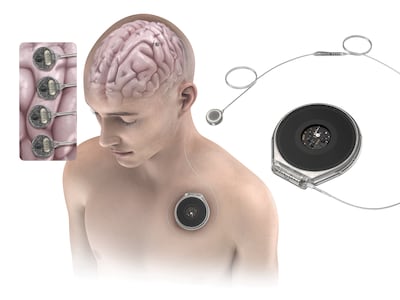
Paradromics won FDA clearance to test its Connexus BCI in two people with severe speech impairment. The 2026 trial will assess whether the device can decode speech in real time.
Ceribell’s algorithm analyzes EEG data collected through a headcap equipped with sensors can identify subclinical events unnoticed without continuous monitoring.
Medtech funding in 2025 sees a trend of fewer, yet larger deals with total funding of $3.6bn in Q1, $2.6bn in Q2 and $2.9bn in Q3, said LSI’s Nick Talamantes. In 2026, he expects a continued investor focus on more mature firms in areas of oncology, cardiology and neurology.
BrainsWay advanced its Deep TMS platform with an FDA labeling expansion for adolescent MDD and the launch of a 200-patient alcohol use disorder trial. The company posted strong Q3 growth and is pursuing new indications and at-home neuromodulation through its Neuralief investment.
Brain-computer interfaces advance toward trials and commercialization, Oura pushes for FDA-cleared blood pressure monitoring, and regulators weigh AI’s expanding role in mental health and diagnostics amid rising safety concerns.
Neurotech start-up Subsense started proof-of-concept studies in mice hoping to show by late 2026 that its nonsurgical, nanoparticle-driven brain-computer interface can read and stimulate neural activity and treat conditions such as Parkinson’s and epilepsy.
INBRAIN teamed up with Microsoft to apply agentic AI to analyze real-time brain data and eventually recommend programming like a “mini-neurologist,” said CEO Carolina Aguilar. It also seeks to enable scalable deployment of INBRAIN’s graphene-based technology and potential joined research.
Synchron raised $200m in series D funding to support pivotal trials in 2026 as well as commercialization of its Stentrode BCI. It also announced plans for a next-gen high-channel whole-brain interface and a new engineering hub in San Diego.