Cybersecurity
Chatbots can churn out valuable information to patients and give much needed assistance to healthcare personnel. But their mistakes can lead to significant patient harm, which is why ECRI ranks their misuse as the top health technology hazard for 2026.
Confido Health has raised $10m in Series A funding to expand its AI voice agent from patient scheduling into referrals, prescriptions and billing, bringing the total collected to $13m. CEO Chetan Reddy predicts in the next decade, AI use for admin tasks in healthcare will become mainstream.
The American Medical Association says years of advocacy have resulted in Medicare policy changes that are a big win for physicians and patients.
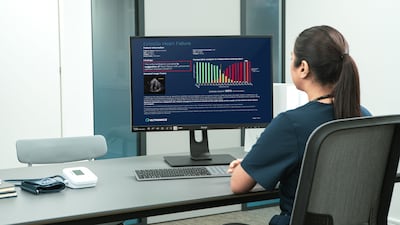
Ultromics has secured $55m in Series C funding to advance its AI tool, EchoGo, which detects undiagnosed heart failure. The company's focus on diseases with available treatments resonated with investors.
The Centers for Medicare and Medicaid Services has launched a new program the agency says will allow patients easier access to their health data. More than 60 companies have already signed on.
The US FDA has issued an updated final guidance document on cybersecurity considerations for medical device manufacturers that replaces a previous final guidance the agency issued in 2023.
Now that the US FDA has chosen not to appeal a March ruling effectively killing the agency’s efforts to regulate lab-developed tests as medical devices, will the agency adopt a different strategy to flex its regulatory muscle?
Congress has launched an inquiry into 23andMe amid privacy concerns following its bankruptcy, particularly regarding the potential sale of sensitive user data. Additionally, a Cybernews report gave 40 DNA testing firms an average cybersecurity grade of D, citing widespread vulnerabilities and data breaches, along with inadequate public information about their security practices.
AI systems used in healthcare are vulnerable to adversarial cyberattacks, which are a growing concern, said Atif Azad, a professor of AI at Birmingham City University. Azad’s research group has developed a method that trains AI to become more resilient to cyber threats through the use of random image adjustments.
Enovis has named veteran medtech leader Damien McDonald as its new CEO effective 12 May as the orthopedic company reaffirms first-quarter 2025 revenue guidance of between $555m and $563m. Medtech Insight spoke with Tim Czartoski, Enovis’ president of US surgical and global product and enabling technologies, about the firm’s growth strategy and innovation plans.
In this week’s Digital Health Roundup, Medtech Insight’s Marion Webb highlights her conference coverage from CES, HIMSS, AAOS and LSI including Exec Chats with Gary Guthart, CEO of Intuitive Surgical, and Arcadia’s chief strategy officer Aneesh Chopra. Brian Bossetta highlights a recently FDA-cleared alert system that sends vital signs to clinicians. Elizabeth Orr discusses FDA warning letters sent to Exer Labs for exceeding marketing claims under what is allowed under the device’s 510(k) clearance. Shubham Singh discusses how Roche's unveiling of its next-generation sequencing (NGS) prototype challenges Illumina. The SBX technology is set to compete directly with Illumina’s NovaSeq and NextSeq platforms.
Medtech Insight sat down with Intuitive Surgical CEO Gary Guthart at the recent LSI USA conference to discuss the full launch of the new da Vinci 5 robotic system and planned digital enhancements. Guthart also offered his views on health care interoperability, AI regulation, outpatient surgeries, autonomous robots, and how the company is harnessing technology to shape the future of robotic surgery.
23andMe has filed for Chapter 11 bankruptcy and announced the resignation of founder and CEO Anne Wojcicki. Privacy concerns surrounding the sale of its extensive genetic database remain after a 2023 data breach exposed data from some 7 million users. Experts warn that bankruptcy proceedings could lead to consumer data being sold, raising questions about legal protections and consumer rights.
Barcelona-based accelerator S2 Xpeed is driving the rapid growth of medtech and hardware start-ups in Europe. Operating under a "sweat equity" model, the program helps early-stage companies move from prototype to manufacturing readiness in exchange for equity. This month, the accelerator will add a fourth cohort of 10 more start-ups, five of which are in the medtech space.
ModMed unveiled its AI-powered ambient listening and clinical documentation software for orthopedic clinics at AAOS, ahead of its planned late-summer release. The health care software provider aims to create “the AI-powered practice” for specialty clinics to reduce clinicians' administrative burden and streamline clinical workflows.
Medtech Insight sat down with Arcadia's chief strategy officer Aneesh Chopra to discuss interoperability, industry standards and the future of health care data and AI.
Google Cloud launches new generative AI capabilities in Vertex AI for health care, allowing clinicians to gain access to multimodal data such as images and data to help with decision-making.
A recent webinar highlighted the need for manufacturers to monitor, manage, and address software vulnerabilities under FDA cybersecurity policy. Important elements include a thorough software bill of materials, coordinated vulnerability disclosure, and maintaining system security.
In this week’s Digital Health Roundup, Medtech Insight’s Marion Webb brings highlights from Deloitte’s 2025 Life Sciences Outlook Report with medtech leaders’ forecasting significant investments in AI/GenAI. Brian Bossetta talks about the US FDA’s newly formed Digital Health Advisory Committee. Elizabeth Orr highlights the pros and cons of penetration testing for cybersecurity and talks about the US FDA’s final guidance on pre-determined change control plans. Natasha Barrow highlights UK MHRA's AI Airlock Pilot program and MANIFEST.
Penetration testing, which involves ethical hackers identifying vulnerabilities before malicious actors can exploit them, is crucial for securing medical devices against cyber threats. The process is essential for compliance with regulations, preventing financial losses, and ensuring patient safety, cybersecurity experts say.