Surgical Procedures
Medtronic has agreed to acquire Cathworks for up to $585m, converting a long-standing partnership into full ownership as it expands its footprint in coronary physiology and AI-enabled cardiovascular tools.
The US FDA says Abiomed has reported 22 serious injuries linked to an issue with some of its temporary heart pumps that provide support to patients with acute right heart failure.
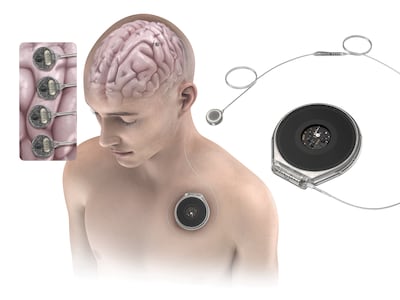
INBRAIN unveiled a bidirectional "rice-sized" BCI chip partnership, Merck commercialization progress and new speech-decoding trial in France as it advances its graphene-based cortical interface toward commercialization, pending regulatory clearance.
In this final part of a three-part series, Medtech Insight spoke with a neuroethicist and the first person in a trial using a BCI implant for stimulating hand movement. This story explores ethical considerations that arise when projects can no longer support patients with implanted devices.
BioStem Technologies’ buyout of BioTissue Holding’s surgical and wound-care business adds cryopreserved and sterile technologies, Cryotek and SteriTek, and a direct sales force focused on acute care settings.
Abbott’s newly CE-marked TactiFlex Duo dual-ablation catheter used for treating AFib is competing against products already introduced by Boston Scientific, J&J and Medtronic.
Amid the spectacle of humanoid robots at CES 2026, Swedish medical simulation company Surgical Science opted for a quieter pitch for its suitcase-sized robotic surgery simulator aimed at taking training out of the OR.
The MAGiC catheter works with Stereotaxis’ robotic magnetic navigation technology, which allows physicians to steer catheters through the heart with precision and stability.
The US FDA’s General Hospital and Personal Use Devices Panel of the Medical Devices Advisory Committee convened Wednesday to discuss germicidal ultraviolet (GUV) devices as a mode of disinfection, a technology that has emerged since the COVID-19 pandemic.
Xeltis secured €50m to scale and market its aXess vascular access graft, a bioresorbable scaffold that transforms into a patient’s own living vessel. With EU regulatory review underway and US trials advancing, Xeltis sees new US Medicare rules as a major opening for alternative treatments.
The US FDA says Olympus has updated its instructions for a device used in many endoscopic procedures after reports of serious injuries. The class I recall follows the FDA blocking imports of other scoping devices from the Japanese firm earlier this year.
Medtronic won FDA clearance for its Hugo surgical robot for urologic procedures, which Wiliam Blair analyst expects will draw interest from physicians. But he also says that Intuitive Surgical will remain the clear dominant player.
Paradromics won FDA clearance to test its Connexus BCI in two people with severe speech impairment. The 2026 trial will assess whether the device can decode speech in real time.
Medtech Insight spoke with Rambam Medical Center’s Michael Mimouni, who implanted the first 3D-bioprinted corneal graft in a human patient, about his hopes for Precise Bio’s approach. The patient is part of a Phase I trial evaluating PB-001 in patients with corneal edema.
INBRAIN teamed up with Microsoft to apply agentic AI to analyze real-time brain data and eventually recommend programming like a “mini-neurologist,” said CEO Carolina Aguilar. It also seeks to enable scalable deployment of INBRAIN’s graphene-based technology and potential joined research.
Advocates spanning the spectrum of women’s health met in Manhattan to discuss the gender disparities that remain in healthcare and how public policy can correct them and the enormous ROI investors in women’s health can potentially reap.
GT Medical says interim results from a clinical trial studying an innovative surgical procedure to treat brain tumors show promise for patients with newly diagnosed operable brain metastases.
At the MedTech Conference, the CEOs of Stryker, Hologic and Insulet said during a panel AI is now central to their business strategies, from creating new medical devices to streamlining workflows and administrative tasks. Stryker’s CEO Kevin Lobo plans a business unit focused on AI-enabled tech.
Successful implantations of a novel transcatheter mark a major step forward in treating congestion in heart failure, according to the California-based company that developed it. The procedure was part of a feasibility study evaluating the device’s safety.
The US FDA has issued a safety alert regarding an Abiomed recall of Automated Impella Controllers, the primary interface for pumps that help to reduce demand on heart’s left ventricle in heart failure patients.