AI advisers could eventually guide consumers toward health products based on personalized needs rather than traditional marketing, IQVIA Consumer Health suggests. But brands will still need to balance data‑driven recommendations with the human appeal of in‑store discovery and self‑care experiences.
Umbrella branding in OTC medicines supports responsible self‑care by helping consumers navigate choices, build trust and confidence, and access reliable health information, countering regulators’ long‑standing focus on risks rather than benefits, says PAGB CEO Michelle Riddalls.
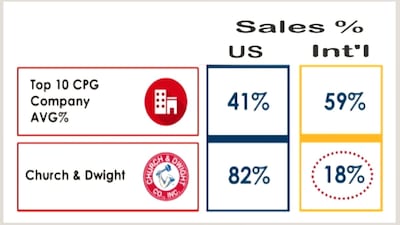
C&D starts 2026 with expectations to boost sales of latest acquisition, Touchland scented hand sanitizer, after halting sales of Flawless hair removal/nail care and Waterpik showerhead products and selling Spinbrush power toothbrush brand as well vitafusion and L’il Critter gummy supplement lines.
A round-up of the latest European people moves: Angelini hires external affairs chief; Pierre Fabre appoints impact and reputation officer; Boots changes leadership at the top.
Both companies announced that during special meetings on Jan. 29, their shareholders voted nearly unanimous support for Kimberly-Clark’s $48.7bn acquisition proposed in November at $3.50 per share plus 0.14625 share for each Kenvue share.
A round-up of the latest European people moves: Dr C. Soldan names new managing directors; EU Specialty Food Ingredients changes secretary general; ASA gets independent reviewer.
Wonderbelly reached market in 2022 and has notched nationwide footprint through major retailers over the years, Target stores starting in March 2023, CVS since June 2024 and Walmart since May 2025.
Key divestments have given the UK's Venture Life the financial firepower to pursue M&A this year, with a number of options under review across categories such as women's health and energy management.
“We remain fully committed to the integrated growth strategy that has enabled us to deliver significant growth and value creation, over the better part of the past decade,” says CEO Shailesh Jejurikar as firm reports net sales up 1% in latest quarter.
Round up of latest European health & wellness people moves: Angelini Pharma gets new CEO; Stada names government affairs chief; NèreS adds to board.
Consumer health companies can help accelerate the shift to prevention as employers and through innovation and cross-sector partnerships, unlocking a potential £42bn in savings for the UK National Health Service.
While it's not clear that it will, if the tech bubble bursts, innovation and investment in consumer health could slow, according to Euromonitor 's Magda Starula. Meanwhile, climate change is reshaping allergy and respiratory needs, creating opportunities for industry.
Executives from Opella, Haleon, Bayer, Kenvue, Cooper and Maxwellia anticipate a year of rapid growth in self‑care and personalised health, driven by digital tools, smarter OTC access and evidence‑led innovation across Europe and beyond.
Futura Medical "delighted" with early feasibility study results for topical OTC treatment WSD4000.
A round-up of the latest health & wellness people moves: Haleon adds chief growth officer role; DSM-Firmenich names HR chief; SkinBioTherapeutics makes additions to its board.
Round up of latest European people moves: Karo makes additions to corporate team; Haleon names Portugal head; Pharma Deutschland adds experience.
Consumer health companies should use clear, science-based messaging across multiple channels and technologies to make health information accessible and reduce reliance on unregulated online sources, advises Deloitte.
AI is rapidly reshaping consumer health advertising across Europe, supporting creative work, compliance checks and regulatory oversight, according to a recently published report by UK consumer healthcare industry association, PAGB.
Reckitt can now focus all of its resources on the consumer health and hygiene markets after divesting its home care business to Advent International.
Consumers are increasingly seeking health and wellness solutions that combine natural ingredients with scientific credibility and technological innovation, reflecting a shift from treating illness to embracing prevention, according to the latest trends report from UK retailer Holland & Barrett.