Marketing & Advertising
AI is rapidly reshaping consumer health advertising across Europe, supporting creative work, compliance checks and regulatory oversight, according to a recently published report by UK consumer healthcare industry association, PAGB.
The trophies have been presented at the OTC Marketing Awards 2025 - but what did the judging panel have to say about the winning and highly-commended entries?
Winners across 12 categories were announced at the OTC Marketing Awards 2025 on 20 November, with Opella crowned OTC Company of the Year.
Karo says adding MTick certification to selected products helps menopausal women “make confident, informed choices when they need it most” and also represents a “significant business opportunity.”
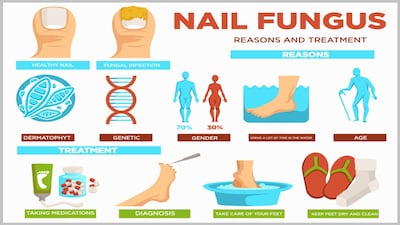
National Advertising Division says XF Agencija permanently discontinued benefits and efficacy ad claims for Lunavia undecylenic acid 25% nail pen challenged by Advantice Health, marketer of Kerasal fungal nail renewal brand.
P&G expands its WICK range with a new natural line of cough syrups; Angelini introduces a guideline-compliant Tantum Verde Duo throat spray; Zentiva adds a children’s fever product to its Ibuflam brand.
Bayer’s EMEA e-commerce and commercial director, Andrew Parker, and Karo Healthcare’s global skin health category director, Sally Perry, discuss how the consumer health industry can meet today's “connected consumers” on their omnichannel shopping journey.
HBW Insight reports from the opening session of Lynx2Market’s PharmaSynergyOTC event in Rome, Italy, featuring IQVIA Consumer Health, Euromonitor International and Amazon.
California-based Primally Pure Skincare is referred to the FTC after failure to comply with a National Advertising Division inquiry into claims its non-nano zinc oxide sunscreen is '100% poison free SPF’.
P&G provides reasonable basis in NAD review for Crest 3D Whitestrips claims on labeling featuring numbers from 4 to 34 to indicate the “Levels Whiter” users’ teeth will appear. NAD says GuruNanda’s population in survey contesting P&G claims was “a fatal flaw.”
P&G supports brand and performance claims for Crest Pro-Health Detoxify toothpaste, NAD concludes, but will modify a fluoride claim following review on a challenge by GuruNanda.
What's going on with the EU Green Claims Directive? HBW Insight speaks to the European Commission, Parliament and Council to find out why trilogue negotiations seem to have stalled.
Eyelids have the "thinnest, most sensitive skin on the body" and are "highly prone to dehydration," which is why Scope has launched new Optase Life Sensitive Eye Daily Renewal Cream.
P&G provided sufficient support for all challenged claims, including the “Gum Detoxify” brand used on the product label and in advertising on the website and in magazine detail pages.
Pharma Deutschland is one of 21 German associations calling for trilogue negotiations in relation to the EU Green Claims Directive to be suspended while a “comprehensive and independent written impact assessment” is undertaken.
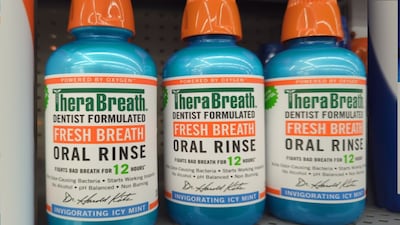
Challenged advertising, which appeared in a TV commercial, in-store displays and advertising on social media, included express claims “It’s a better mouthwash. I guarantee it.” and “I guarantee it. – ‘Dr. Harold Katz.’”
Navamedic has launched Eroxon in Danish pharmacies giving men in the country OTC access to an ED treatment for the first time.
Italy's Alfasigma has expanded its operation in Germany with the creation of a new Consumer Healthcare unit. Led by Rüdiger Hoppe, the business is rolling out OTC medical devices and dietary supplements in the gastrointestinal category.
Maxwellia's “bold, honest, and completely unfiltered” campaign for Evana menstrual health brand tells the story of a woman dealing with the realities of a heavy period, showing “real blood, real leaks.”
Pirilieve Hayfever Relief Tablets contain 120mg fexofenadine hydrochloride, which the firm notes is an active ingredient that was “previously only available behind pharmacy counters.”