Regulation
The European Commission’s recent environmental omnibus failed to address industry concerns on wastewater cost allocations, and legal pathways are expected to be the way forward.
Private Label Skin Care Inc. has violated Current Good Manufacturing Practice regulations for pharmaceuticals, according to the agency.
FDA’s draft guidance on mandatory cosmetic recalls under MoCRA signals how the agency plans to exercise its new recall authority and urges companies to strengthen compliance, recall readiness and rapid response procedures.
The US FDA issued a report on PFAS, as mandated by Congress under the Modernization of Cosmetics Regulation Act, on Dec. 29, concluding that the safety of most PFAS cannot be established due to a lack of available data.
Former agency officials now representing industry worry that a deregulatory bent could be driving "Simple Reform" plan to merge all medical product and clinical research inspectorates and that specialist expertise gained in 2017 "Program Alignment" initiative will be reversed.
The Colorado Department of Public Health and Environment approved Circular Action Alliance’s final plan for the state’s Extended Producer Responsibility program on Dec. 9.
Sunscreen products industry and public health advocacy groups have been critical that FDA has not approved a new filter since 1999 even as countries in Europe and other regions allow using numerous additional ingredients in sunscreens.
CDER Office of Generic Drugs publishes MaPP for prescription-to-nonprescription switches and ANDAs to explain regulatory responsibilities for makers of generic copies of reference listed drugs approved for OTC switch.
The US FDA announces in a Nov. 28 Federal Register notice that it will withdraw its proposed rule under MoCRA for testing asbestos in talc-containing products, but remains committed to meeting its statutory requirements under the law.
Congress’ reauthorization of OMUFA for five years includes measures that promote non-animal testing for sunscreen, a broadening of the evidence that can be used to support safety and additional funding for MoCRA.
Consultants urge packaging producers to forecast and budget now for the fees of state EPR programs, particularly as fees from the Oregon program hit and surprise many firms and expose gaps in internal budgeting and planning. The topic was discussed at IBA’s Cosmetics Convergence 2025 conference.
The US FDA’s decision to move its long-awaited cosmetic GMPs rule to ‘Long-Term Actions’ under MoCRA likely delays a final rule for at least five years, raising the risk of increased state regulation and private litigation, say attorneys speaking during the 2025 PCPC Science Symposium & Expo
Regulations likely holding consumer product firms’ attention include the Plastic Pollution Prevention and Packaging Producer Responsibility Act, SB 54, scheduled to become effective by 2027. “Easily the most significant, extended producer responsibility law ever to pass in the world,” says lobbyist
A Senate committee is investigating leadership changes at the US Centers for Disease Control and Prevention. While the primary focus is vaccine policy, the first hearing suggested the probe could raise questions about FDA independence.
Retailers selling beauty products in the state of Washington who plan to dispose of unpurchased products by Jan. 1 must follow the state's Dangerous Waste Reduction law, which categorizes waste generators as small, medium or large.
Continuing work includes “for cause and certain surveillance inspections of regulated facilities” and “criminal enforcement work and certain civil investigations” as White House mulls layoffs rather than furloughs during federal government shutdown.
The Washington Department of Ecology will begin collecting feedback for its rulemaking on lead limits for cosmetics under the Toxic Free Cosmetics Act early next year, and industry’s response throughout the process will be critical to determine the outcome.
Recent FDA warning letters hit typical problems in consumer health space such as manufacturing quality control deficiencies, but also note honey offered for eye infections and nasal spray to enhance cognitive function.
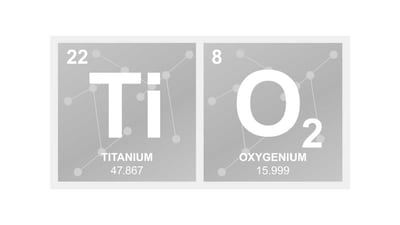
In separate cases in California and Europe, courts reached similar conclusions regarding the potential carcinogenicity of titanium dioxide in respirable form and its impact on regulations.
Washington regulators announce restrictions on intentionally added formaldehyde releasers take effect Jan. 1, 2027, with retailers having until Jan. 1, 2028, to sell through existing stock.