Regulation
“FDA’s proposed GRAS rule would move GRAS closer to the NDI model in at least one key respect, which is mandatory notification,” says attorney Ashish Talati. Proposed rule isn’t expected to require removal of ingredients available through self-GRAS processes.
Changing self-GRAS regulation, providing NDIN safety and identity guidance and modernizing supplement industry regulatory framework are on FDA’s “2026 priority deliverables,” but not requirement for public listing of all supplements available.
Former agency officials now representing industry worry that a deregulatory bent could be driving "Simple Reform" plan to merge all medical product and clinical research inspectorates and that specialist expertise gained in 2017 "Program Alignment" initiative will be reversed.
Third-Party Audit Results ‘Definitely The Direction’ For FDA Supplement Facility Inspection Planning
“There's a lot of third-party audit information out there. Could we take that and apply it into our inspectional planning process?” says Office of Dietary Supplement Programs Director Cara Welch.
FDA looks to Congress for authority to require registration for all supplements available for sale in US for public-facing product listing but no authority can produce list of dietary ingredients available pre-DSHEA.
Enforcements at facilities in Missouri operated by Shaman Botanicals and Relax Relief Rejuvenate Trading were preceded by administrative detention orders, FDA’s first use of the authority since its reorganization in October 2024.
BRS Analytical Services in St. Louis recalled more than 75,000 cases of redness and lubricating eye drops and committed “to permanently cease production of all drugs.”
Regulations likely holding consumer product firms’ attention include the Plastic Pollution Prevention and Packaging Producer Responsibility Act, SB 54, scheduled to become effective by 2027. “Easily the most significant, extended producer responsibility law ever to pass in the world,” says lobbyist
A Senate committee is investigating leadership changes at the US Centers for Disease Control and Prevention. While the primary focus is vaccine policy, the first hearing suggested the probe could raise questions about FDA independence.
FDA rejects NPAs arguments for clarity on when it recognizes start of investigational new drug programs and what constitutes “substantial clinical investigations” for an IND. Clock starts after FDA met twice-extended deadline to provide answers to NPA’s questions in a 2023 petition.
Continuing work includes “for cause and certain surveillance inspections of regulated facilities” and “criminal enforcement work and certain civil investigations” as White House mulls layoffs rather than furloughs during federal government shutdown.
In change with potential to help supplement firms establish availability of dietary ingredients in US predates their use in drugs, FDA grants argument by NPA and CRN that marketing of ingredients dates to even when they use in supplements may be unlawful under agency regulations.
FDA responds to trade groups' citizen petitions saying it wouldn’t change its policies for when INDs are registered, dates which could preclude subsequent introductions of dietary ingredients, and what constitutes “substantial clinical investigations” in INDs.
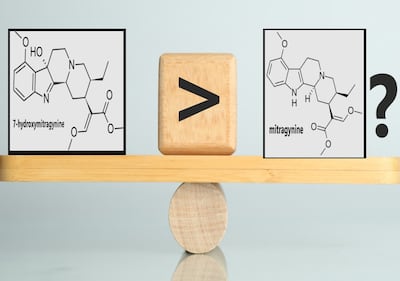
Holistic Alternative Recovery Trust advocacy group for 7-OH use to help people stop using opioids offers arguments from a variety of public health and consumer advocacy groups that scheduling 7-OH “would be a disaster.”
Recent FDA warning letters hit typical problems in consumer health space such as manufacturing quality control deficiencies, but also note honey offered for eye infections and nasal spray to enhance cognitive function.
Ryze Superfoods withdraws claims National Advertising Division asks to review from its ongoing monitoring of consumer products advertising, including that coffee enhances mental acuity and provides other health benefits.
Proposed rule to eliminate self-GRAS without submitting regulatory notification planned seven months after HHS Secretary Kennedy Jr. suggested change. Latest HHS regulatory agenda also includes publication of FDA final rule to deem supplements unapproved drugs when co-packaged with drugs.
AHPA is among commenters on FDA’s proposed post-market assessment tool to determine which chemicals should be subject to further scientific and regulatory scrutiny. HHS proposes nutrition education requirements embedded in pre-medical standards and medical school curricula Integration.
UK CBD food supplement applications from Chanelle McCoy, Cannaray Brands and the European Industrial Hemp Association pass FSA's safety assessment stage and are now out for consultation. After that, only government approval stands in their way, with five years exclusivity the icing on the cake.
Holistic Alternative Recovery Trust says FDA should review data and other information available about the safety of all kratom extracts before it moves ahead on its proposal to recommend Schedule I controlled substance for 7-OH.