Compliance
Agency exercises enforcement discretion on requirement for products to be labeled as containing artificial colors or dyes when those aren’t native to foods or ingredients, including added colors made from natural sources.
FDA Human Foods Program’s list notes Kennedy’s GRAS focus as first item in “Food Chemical Safety” category, saying the agency in 2026 “will publish a proposed regulation to require the submission to FDA of GRAS notices for all substances claimed to be GRAS.”
Only Opella’s own Enterogermina brand was found by a company-funded study to be contamination-free, to contain the claimed content, and show resistance to all tested antibiotics.
UK regulator issues reminder that melatonin is a prescription-only medicine in the UK after the sleep aid is found in a dietary supplement for children.
Germany was more active than any other EU member in the second quarter in submitting notifications to the Commission regarding problematic food supplements.
Three FDA warnings related to supplement manufacturing, labeling or marketing submitted to Amazon or companies selling vitamin, mineral or supplement products on its e-commerce platform since April 2024.
HHS Secretary Kennedy commends food companies for cooperating, but also says, “We have them on the run now and we are going to win this battle. And four years from now, we're going to have most of these products off the market.”
FDA introduced the option because it didn’t have sufficient staff to handle the volume of GRAS submission reviews requested by food and other firms for ingredients. Requiring submissions for all GRAS determinations “would just be an unworkable situation for the food industry if somehow submitting notice, submitting notices for FDA review and concurrence, was required,” says food and drug attorney Federick Stearns.
FDA says “extension affords covered entities the additional time necessary to ensure complete coordination across the supply chain in order to fully implement the final rule’s requirements—ultimately providing FDA and consumers with greater transparency and food safety.”
UK industry association is offering online courses on subjects ranging from UK medicines regulations basics to deep dives on complex topics such as CBD.
Trade group ready to work hand-in-hand with agency and other supplement industry stakeholders on potential regulatory changes or improvements, says president and CEO Steve Mister. “None of them are upsetting the basic balance of things that DSHEA was attempting to do, but there are things with 30 years that we've identified that need to be kind of fixed.”
New Jersey Assembly passed bill amended to expand extensively in defining which products, or ingredients, would be subject to a ban on sales to consumers younger than 18.
In first of two articles from recent interview, president and CEO Steve Mister discusses examples the trade group provides for self-regulation, “where people or companies who might not do it on their own because it would put them at a disadvantage if they were the only ones.”
The Federal Trade Commission’s Consumer Protection head Sam Levine says bipartisan leaders at the state and federal levels are taking on consumer protection issues like never before and using the FTC’s approach as a model for legislation.
Green Pharmaceuticals’ SnoreStop Nasal Spray, previously marketed as “NasoSpray,” still is available even though agency officials on multiple occasions for a month recommended a recall after an April inspection found “gross microbial contamination” in one lot.
Reorganization creating in Human Foods Program makes the work of additional staff available for dietary supplement office programs. FDA’s “got a little tiny workforce” for “a huge industry,” says Commissioner Robert Califf.
Report from European Commission's Alert and Cooperation Network finds EU consumers are being deceived by companies marketing supplements making unauthorized health claims and containing unapproved ingredients.
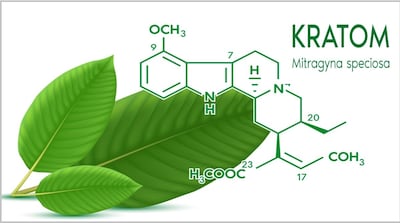
Week after publishing safety alert about OPMS Black Liquid Kratom “linked to serious adverse health effects, including death,” FDA announced market research “to understand and characterize emergent risk/safety and perceived benefits reportedly linked to kratom and psychedelics.” But it withdrew the study 10 days later.
Dietary supplements containing unauthorized novel foods were reported to the European Commission by national regulators on around 40 occasions in the second quarter of 2024.
As it has every year since FSMA was passed in 2011, FDA doesn’t plan to impose reinspection fees until it publishes guidance for small businesses to request reductions. FY2025 budget proposal includes plan “to re-structure the fee programs to make it more administratively feasible to operate.”