Legislation
A new bill introduced in the US House of Representatives seeks to curb the patchwork of packaging regulations for consumer products in different states with a federal standard.
The Council of the European Union stakes out its position on the Omnibus VI package, one that adopts a more precautionary approach and puts industry on edge.
But the Senate’s reduction would not be as much as the House of Representatives and President Trump proposed earlier this year, which is likely a win for the agency.
Multiple microbeads measures in California could impact how manufacturers selling products in the state approach reformulation.
OMUFA reauthorization bill including amendment with sunscreen regulation provisions passed by Senate committee, which also approved other provisions not included in House OMUFA bill.
As stakeholders and lawmakers pin their hopes on OMUFA reauthorization as a vehicle for enacting sunscreen regulatory changes, Senate version of the bill could be the ticket after a sunscreen-specific amendment withdrawn in the House.
Committee voted 51-0 to approve five-year authorization of FDA program user fee program needed to support regulatory pathway for large majority of nonprescription drugs available in the US with amendment to expand stakeholder engagement with FDA.
What's going on with the EU Green Claims Directive? HBW Insight speaks to the European Commission, Parliament and Council to find out why trilogue negotiations seem to have stalled.
FDA would receive more funding for fiscal year 2026 in the Senate appropriations bill than its House counterpart, including $10m for unannounced foreign inspections.
The Safer Beauty Bill Package comprises four federal bills introduced in the House that aim to close regulatory ‘gaps’ in cosmetics oversight left by the Modernization of Cosmetics Regulation Act.
The US House of Representatives passes legislation requiring manufacturers of non-flushable wet wipes to label products as ‘non-flushable," a move endorsed by the Personal Care Products Council.
Lawmakers in California heeded calls from industry to change proposed legislation to avoid a wider ban on microplastics in personal care products.
Four co-chairs of the House of Representatives’ Skin Cancer Caucus have introduced the Safe Sunscreen Standards Act to direct FDA to establish clearer, ‘more flexible’ standards for evaluating sunscreen ingredients.
A US House bill would give the FDA $33.1m more in budget authority than requested by the Trump Administration for fiscal year 2026. The measure was sent to the full House Appropriations committee on a party-line vote.
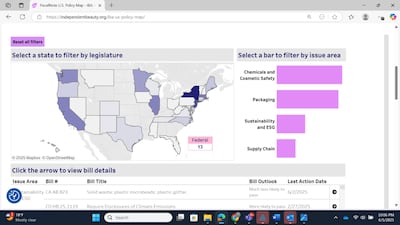
The Independent Beauty Association has launched an interactive Policy Map tracking state and federal bills and filtering by state or topic, pinpointing bills of great priority.
The US cosmetics industry, particularly small- and medium-sized businesses, should reach out to their representatives in Congress to encourage pressure on the US Food and Drug Administration to implement cosmetics reform, despite agency staff cuts.
Preliminary registration data released by FDA offers a first glimpse of the Modernization of Cosmetics Regulation Act’s impact on information the agency has at hand.
The Washington State Department of Ecology publishes ‘Interim Policy on Lead in Cosmetics’ which provides safe harbor options for cosmetic products struggling with the 1ppm limit under the state’s Toxic Free Cosmetics Act, while the department gathers information under a newly opened rulemaking to ‘identify a feasible approach to regulating lead in cosmetic products.’
The Washington Department of Ecology hasn’t backed down on its targeting of formaldehyde-releasing preservatives under the state’s Toxic-Free Cosmetics Act, as industry still awaits a draft final rule. In a recent webinar, attorney Angela Deisch of Amin Wasserman Gurnani, LLP said the department has also not provided clarity on penalties under the law, which goes into effect 1 January.
The FDA's current leader, whose term will end with Donald Trump’s second inauguration, also described three qualities the agency’s next commissioner will need to succeed, including "believing that there is such a thing as expertise."