Recalls
When FDA warned Agebox about selling iKids-Growth IGF-1 Support supplements as unapproved drugs, agency along with CDC had for around a month been investigating outbreak of infant botulism linked to ByHeart formula.
During webinar hosted by Sedgwick, regulatory experts discussed how firms can optimize engagement with FDA, especially when it comes to communicating recalls and corrective actions.
“We have rarely, if ever, enforced this requirement” FDA says announcing enforcement discretion regarding requirement for disclaimer about supplement structure/function and other label claims to be printed on every panel of a product’s package.
“Company employee diverted legitimate packaging and customer information to distribute counterfeit, adulterated product,” says Green Lumber. It’s “working cooperatively with FDA and law enforcement to address this matter.”
UK regulator issues reminder that melatonin is a prescription-only medicine in the UK after the sleep aid is found in a dietary supplement for children.
Kyle Diamantas was a partner with the Jones Day firm when he was tabbed as acting deputy commissioner to lead the FDA’s Human Foods Program, established in the agency’s reorganization which became effective in October.
Streamlined process for reporting problems is key piece of “unified Human Foods Program” which officially launched on 1 October, as Commissioner Robert Califf says, “a new model for field operations and other modernization efforts.”
Brands also making market moves as lawmakers consider legislation instructing Transportation Security Administration to provide guidance to minimize risk for contamination of baby formula and related pediatric nutritional products.
Green Pharmaceuticals’ SnoreStop Nasal Spray, previously marketed as “NasoSpray,” still is available even though agency officials on multiple occasions for a month recommended a recall after an April inspection found “gross microbial contamination” in one lot.
As it has every year since FSMA was passed in 2011, FDA doesn’t plan to impose reinspection fees until it publishes guidance for small businesses to request reductions. FY2025 budget proposal includes plan “to re-structure the fee programs to make it more administratively feasible to operate.”
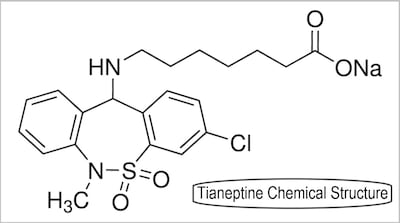
Senate majority whip asks four supplement industry trade groups for “a written plan to work with responsible supplement manufacturers to remove tianeptine and other dangerous or illegal ingredients from the supplement market.”
Nearly 1,300 rodents exterminated at Arkansas warehouse after FDA investigation found lack of climate control in store of food and OTC drugs requiring dry, room-temperature conditions. Firm pleads guilty in largest monetary criminal penalty in US food safety case.
Perrigo doesn’t attempt to diminish significance of challenge to regain sales growth in formula market as it resets its plants. Analysts see formula business as only current impediment to stronger results.
Reps. Panetta and Pfluger introduce Scheduling Tianeptine and Analogues Now to Defend Against Emerging Opioids Act to make tianeptine a schedule III controlled substance a day after FDA posts latest alert to warn consumers against using products containing ingredient known as “gas station heroin.”
Warning references consumer alerts FDA published in February and August about products agency officials purchased on Amazon.com and determined to contain undisclosed sildenafil or tadalafil, PDE-5 inhibitors approved for use in Rx drugs with an ED indication.
Agency proposal, now under HHS review, further reduces chances of losing another whistleblower complaint by taking ORA out of the loop, while proposing a new name for its slimmed-down field organization: Office of Inspections and Investigations. Meanwhile, an agency veteran, Michael Rogers, will lead the revamped organization as associate commissioner.
Tianeptine isn’t approved as drug in US nor allowed for use as dietary ingredient or food additive. FDA has warned multiple firms selling tianeptine-containing products and federal prosecutors recently announced guilty plea from man who sold the drug to consumers.
Higher costs and interest rates due to inflation as well as higher credit card debt point toward consumers spending less in areas including health, personal and household care products. C&D is “well-positioned for this trade down” with 40% of its portfolio in value products, says CEO Matthew Farrell.
Recall starts after products across OTC indications, including at-home pregnancy and marijuana tests, “were stored outside of labeled temperature requirements” and “inadvertently shipped to certain stores” in 23 states.
Biomic Sciences recalls all lots of ION* Sinus Support and ION* Biome Sinus after FDE testing found microbial contamination causing reasonable probability of life-threatening adverse events for patients or people who recently underwent nasal or sinus surgery.