Retail
The global allergy market is “expanding again but on new terms, driven by a mix of regional strength, evolving consumer behaviors, and an increasingly diverse competitive field,” according to the latest IQVIA Consumer Health analysis.
Women in England are now eligible to access the morning after pill for free from their community pharmacy. Maxwellia CEO Anna Maxwell welcomes the move, but warns that the service must be balanced with OTC options because “waiting, even for a free service, may simply not be an option.”
Tmall’s business development manager, Aline Hu, and founder of cross-border e-commerce specialist QIVA Global, Ellie Adams, offer advice to multinational consumer healthcare players looking to enter the potentially lucrative and growing Chinese consumer healthcare import market.
Bayer’s EMEA e-commerce and commercial director, Andrew Parker, and Karo Healthcare’s global skin health category director, Sally Perry, discuss how the consumer health industry can meet today's “connected consumers” on their omnichannel shopping journey.
HBW Insight reports from the opening session of Lynx2Market’s PharmaSynergyOTC event in Rome, Italy, featuring IQVIA Consumer Health, Euromonitor International and Amazon.
“Our new range of own label medicines have now launched in store and that’s why we’ve partnered with PAGB, who provide expert regulatory and policy guidance and support in an evolving and complex sector,” explains the UK retail chain, which has become PAGB's 100th member.
First Insight survey with 1,267 US consumers showed 44% are more likely to try a private label product “marketed as a dupe of a high-end product.” Providers of OTC drugs as well as other medical products in US would tread on thin regulatory ice with the practice.
Terclara - Moberg’s proprietary topical formulation of terbinafine for fungal nail - has established itself as the market leader in Norway just months after its OTC launch. The firm is now plotting its expansion to additional EU markets.
Just under half of Austrian pharmacists surveyed by Elpato Medien are “convinced that the OTC business will ensure the survival of their pharmacy.”
Almost two thirds (62%) of respondents to an online survey said they would rather buy non-prescription medicines in a local pharmacy than via a mail-order pharmacy, Pharma Deutschland reports.
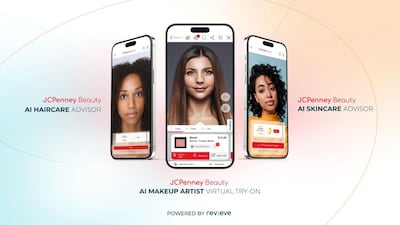
Retailer JC Penney Company, Inc.’s use of Revieve Inc.’s virtual try-on and beauty advisor tools has driven significant increases in online engagement, average order rates and conversion rates for makeup, skin and hair care versus the retailer’s other e-commerce products.
Although FDA concluded OTC oral phenylephrine is ineffective as a nasal decongestant in doses up to highest studied level, federal preemption precludes challenges to agency’s regulation of labeling for phenylephrine and other Rx and OTC drugs, according to recent decision in New York federal court.
Firm’s future, as a separate business nearly 90% owned by Bausch Health, or as a standalone with different ownership or one not controlled by a majority owner, claims high profile in analysts’ comments and predictions. B+L is in the driver’s seat, they say.
Reckitt backs up prediction for strong sales during current quarter with advertising featuring a beauty queen and a line extension for Mucinex, one its strongest US consumer health product lines.
Requests for “enforcement actions are not within the scope of FDA’s citizen petition procedures,” CDER says, rejecting petition dosing device firm Parenteral Technologies submitted as it prepares for workshop on Pediatric Research Equity Act requirements for OTC NDA sponsors.
UK pharma also reaches agreement in principle, subject to DoJ approval, to pay $70m to resolve a whistleblower complaint filed by Valisure, the testing lab which in 2019 raised concerns about a potential link between the use of drugs containing ranitidine, a histamine-2 blocker, and cancer.
Regenerative Processing replaces nozzle to prevent backflow for its Regener-Eyes drops but FDA warning states numerous questions about sterility at the firm’s plant and about its procedures and systems for preventing microbial contamination.
After launching Retaine MGD Advanced, OcuSoft says a release by Bruder Healthcare referenced Retaine MGD trademark and statements from a previous OcuSoft announcement about the original product attributed to an optometrist. B+L, Rohto brand and homeopathic firm Relief Products also make US OTC eye care space moves.
Jessica Spence joins as North American president while CFO Dan Sullivan becomes COO and Francesca Weissman, finance and business strategy SVP, adds CFO to her title in latest executive office changes by the marketer of brands including Schick men's and women’s shaving, Playtex, Stayfree, Carefree and o.b. feminine care and Banana Boat and Hawaiian Tropic sun care.
Nonbinding forecast, fourth for the program, includes risks associated with codeine-containing cough medicine as a topic FDA will include in ongoing evaluation of GRASE for pediatric cough cold drug products marketed under monograph for cold, cough, allergy, bronchodilator and anti-asthmatic OTC drugs.